Bayer’s Mirena IUD (intrauterine device) is an implant used to prevent pregnancy for up to five years through the continual release of the hormone levonorgestrel. Although it was supposed to make avoiding pregnancy “simple,” Mirena IUD injuries are becoming a major medical – and legal – concern. Perforated Uterus The latest Mirena IUD lawsuit filed against Bayer Healthcare Pharmaceuticals involves a woman whose uterus was perforated by the device. According to the complaint recently filed in New Jersey Superior Court,… Read More
The Driscoll Firm Legal Blog Archive

Medtronic’s Infuse Bone Growth product was originally marketed as an innovative way to perform spinal fusion surgeries by using a genetically engineered protein to stimulate bone growth in the spine. However, nearly 85% of procedures using Infuse are now off-label – which is causing concern about who’s liable for its related injuries. Who’s Responsible for Infuse Injuries? According to a report by HealthWorks Collective, nearly 85% of procedures using Medtronic’s Infuse Bone Growth product are off-label – meaning they haven’t… Read More

More and more patients are claiming that Medtronic’s Infuse Bone Growth doesn’t work and carries serious side effects. While patient lawsuits against the company are mounting, Medtronic shareholders are also angry. In a recently filed lawsuit, they claim that the company knew about the issues, but downplayed them to inflate stock prices. Medtronic Concealed Known Facts – From Everyone Minnesota based Medtronic, a global medical device company, has seen its share of scandals. In 2007, Medtronic’s Sprint Fidelis defibrillator lead… Read More

Most of the clinical trial work that occurs before a drug is approved by the U.S. Food & Drug Administration is not fully published – often due to “cozy” relationships between pharmaceutical companies and the health care industry. However, one man is trying to change that. If he is successful, it may change the way the drug industry currently operates. Too Close For Comfort That’s how Peter Doshi, a Johns Hopkins University postdoctoral fellow, describes the relationships between pharmaceutical companies… Read More

A new lawsuit filed against the Coloplast Corporation and others alleges that a woman experienced serious transvaginal mesh side effects after using several of the company’s TVM products. Her transvaginal mesh lawsuit joins thousands of others filed in a multi-district litigation (MDL) that is pending in the U.S. District Court for the Southern District of West Virginia. Defective & Dangerous Products That’s how a Texas woman’s lawsuit describes the three transvaginal mesh products that were used to treat her pelvic… Read More

Endo Health Solutions, which recently purchased American Medical Systems, has agreed to pay nearly $55 million to settle allegations that AMS transvaginal mesh (TVM) implants eroded in some women and caused them serious injuries. Settlement Does Not Affect Majority Of TVM Lawsuits Consolidated In MDL Although details of the $55 million settlement haven’t been disclosed, according to Bloomberg News, Endo reported that the deal will “resolve an unidentified number of suits over the company’s vaginal-mesh devices, which include the Perigee,… Read More

The U.S. Supreme Court recently ruled that generic drug manufacturers can not be sued under state law for adverse reactions to their products because it would run against federal laws on prescription medications that are approved by the U.S. Food & Drug Administration. What The Ruling Means In 2011, the U.S. Supreme Court ruled that makers of “branded” drugs could be held responsible for injuries resulting from their products. However, the Court’s decision didn’t tackle the issue of whether makers… Read More

Transvaginal mesh injury lawsuits filed against CR Bard allege that the manufacturer used plastic materials in some of its Avaulta line of implants that were unfit for use in humans – even after Bard’s supplier warned it of the dangers. Plaintiffs Allege Bard Ignored Warnings According to Bloomberg News, recently unsealed court records show the CR Bard used a resin-based plastic in some of its Davol brand transvaginal mesh products. Bard’s supplier, Chevron Phillips Chemical Company and one of its… Read More
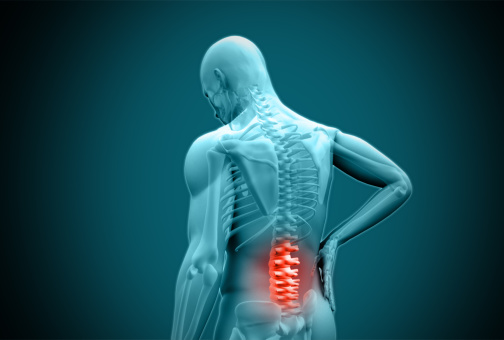
Medtronic’s Infuse Bone Graft has been found to work no better than traditional bone graft surgery. To make matters worse, it has also been linked to serious side effects such as cancer, sterility and infections – causing Infuse Bone Graft patients to question why more research wasn’t done before the product was put on the market. Risks May Outweigh Benefits According to the Bloomberg News, although Medtronic’s Infuse Bone Graft was designed to help bones heal after spinal surgery, its… Read More

Increasing numbers of transvaginal mesh (TVM) injury lawsuits are being filed every day against manufacturers such as C.R. Bard, Johnson & Johnson and its subsidiary, Ethicon. Defendants in the latest TVM lawsuit, filed in the U.S. District Court for the Southern District of West Virginia, include both Boston Scientific and American Medical Systems. Woman Alleges MiniArc Sling & Pinnacle Pelvic Kits Are Defective – And Dangerous According to a recent report in Newsday, the plaintiff in the latest TVM injury… Read More








